The particles which build together the atoms are called subatomic particles. In this article, we will learn about the discovery of electrons, protons and neutrons.
Discovery of Electron- Discharge Tube Experiment
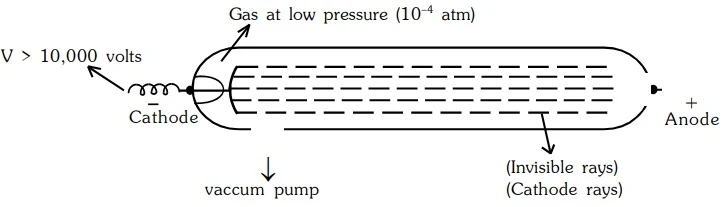
In 1859, scientists studied electricity in gases using a glass tube. They applied a high voltage and noticed invisible rays traveling from the negative electrode (cathode) to the positive electrode. These invisible rays were named cathode rays.
Properties of Cathode Rays
- They travel in straight lines away from cathode with very high velocity ranging from 107 to 109 m/sec.
- A shadow of metallic object placed in the path is cast on the wall opposite to the cathode.
- They produce a green glow when strike the glass wall matter. Light is emitted when they strike the
zinc sulfide screen.

- When a small pin wheel is placed in their path, the blades of the wheel are set in motion. Thus the
cathode rays consist of material particles which have mass and velocity.
- They are deflected by the electric and magnetic fields. When the rays are passed between two electrically
charged plates, these are deflected towards the positively charged plate.
It shows that cathode rays carry negative charge. These particles carrying negative charge were called negatrons by Thomson. The name negatron was changed to 'electron' by Stoney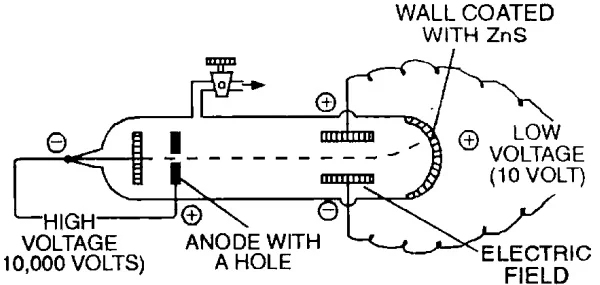
- They produce heat energy when they collide with the matter. It shows that cathode rays posses Kinetic energy which is converted into heat energy when stopped by matter.
- These rays affect the photographic plate.
- Cathode rays can penetrate the thin foil of solid materials.
- Cathode rays can ionize the gases through which they pass.
- The nature of cathode rays is independent of
(a) The nature of cathode and
(b) The gas in discharge tube
Measurement of Charge to Mass Ratio (e/M) for Electron
In 1897, J.J. Thomson determined the e/m value (charge/mass) of the electron by studying the deflection of cathode rays in electric & magnetic fields.
The value of e/m has been found to be –1.7588 x 108 coulomb/g.
- By performing a series of experiments, Thomson proved that whatever gas be taken in the discharge tube and whatever be the material of the electrodes the value of e/m is always the same.
- Electrons are thus common universal constituents of all atoms.
Measurement of Electronic Charge (e) - Millikan's Oil Drop Method
The absolute value of the charge on an e- was measured by R.A. Milikan in 1909 by the Milikan's oil drop experiment.
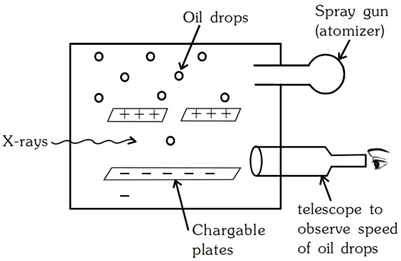
- Oil Droplets: He sprayed tiny oil droplets through a hole in the top plate.
- Electrons Get Stuck: He zapped the air with X-rays, which knocked electrons off air molecules. Some of these electrons stuck to the oil droplets, making them negatively charged.
- Electric Field Control: He could adjust the electric field between the plates by charging them.
- Balancing Act: By adjusting the electric field, he could balance the falling force of gravity on the oil droplet with the upward force of the electric field on the negatively charged droplet. This made some droplets hover!
- Calculations: By measuring how fast the droplets fell and how strong the electric field needed to be for hovering, he could figure out the charge on the oil droplets.
- Smallest Charge Wins: He noticed that all the charges were multiples of a tiny, fundamental value, which he figured was the charge of a single electron. That value was around 1.60 x 10-19 Coulombs (C).
This experiment helped scientists measure the exact charge of an electron, a fundamental building block of our world.
Mass of Electron
Mass of the e-
can be calculate from the value of e/m and the value of e
m = 9.1096 x10–28 g or 9.1096 x 10–31 kg
This is termed as the rest mass of the electron i.e. mass of the electron when moving with low speed.
The mass of a moving e- may be calculate by applying the following formula.

Where v is the velocity of the e- and c is the velocity of light.
When,
- v = c ⇒ mass of e- = ∞
- v > c ⇒ mass of e- = imaginary
Discovery of Proton - Anode rays
Goldstein's Experiment:
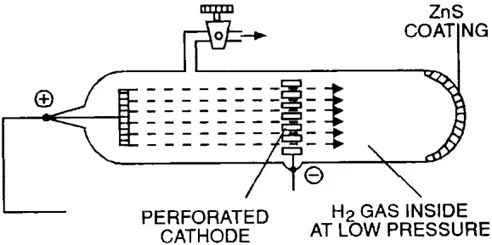
- Goldstein modified a cathode ray tube with a perforated cathode.
- He observed new rays traveling from the anode (positive electrode) towards the cathode (negative electrode) through the holes in the cathode.
- These rays were called canal rays (due to their path) or anode rays (due to their origin).
Thomson's Analysis:
- Positive charge deflection by electric and magnetic fields.
- Mass - cast shadows, deflected by fields (kinetic energy).
- Other properties - similar to cathode rays in some ways.
Key Observation:
- The ratio of charge to mass (e/m) for these positive particles was much smaller than for electrons, indicating a higher mass.
- The e/m value also depended on the gas used, suggesting different types of positive particles.
Discovery of the Proton:
- Thomson measured the e/m value of positive particles in hydrogen (lightest gas).
- This value was the highest observed for any positive particle.
- It was assumed this particle from hydrogen was a fundamental positive particle.
- Rutherford named it the proton in 1911.
Charge and Mass of Proton
- Charge: Equal in magnitude but opposite in sign to an electron (+1.602 x 10-19 C).
- Mass: Calculated from e/m value (1.672 x 10-27 kg or 1.00757 amu).
Discovery of Neutron
In 1920, Rutherford suggested that in an atom, there must be present at least a third type of fundamental particles which should be electrically neutral and posses mass nearly equal to that of proton. He proposed the name for such fundamental particles as neutron.
In 1932, Chadwick bombarded beryllium with a stream of alpha particles. Alpha particles are nothing but nucleus of Helium atom 4He2. He observed that penetrating radiations were produced which were not affected by electric & magnetic fields. These radiations consisted of neutral particles, which were called neutrons. The nuclear reaction can be shown as

- Thus a neutron is a sub atomic particle which has a mass 1.675 x 10–24 g approximately 1amu, or nearly equal to the mass of proton or hydrogen atom and carrying no electrical charge.
- The e/m value of a neutron is zero.
Previous Topic - Dalton Atomic Theory
Next Topic - Thomson Atomic Model
Read Full Chapter - Structure of Atom

